Abstract
Background: Sickle cell disease (SCD) is an inherited systemic disorder characterized by chronic hemolytic anemia and recurrent vaso-occlusion, which can lead to acute and chronic complications, disability, and early mortality. Patients with SCD are hospitalized frequently and most commonly for vaso-occlusive crises (VOCs). Furthermore, most will require a blood transfusion for prevention or acute management of complications, with >90% of adults receiving ≥1 transfusion in their lifetime. Voxelotor (Oxbryta ®) tablets are indicated for treatment of SCD in adults and adolescents aged ≥12 years. Emerging evidence shows that voxelotor, an oral, once-daily sickle hemoglobin-polymerization inhibitor, may improve the clinical symptoms of SCD and reduce VOC rates as well as the need for transfusions. This study sought to assess the real-world impact of voxelotor treatment on the rates and management of SCD-related complications.
Methods: Medical and pharmacy claims data for patients aged ≥12 years with SCD who initiated voxelotor therapy between November 2019 and March 2021 were procured from the Symphony Health claims database. Patients with ≥1 year's data before the index date (date of the first voxelotor claim for each patient) were included in the analyses. Baseline demographic and clinical characteristics were summarized using descriptive and inferential statistics. Annualized rates per patient-year (PPY) for transfusions, VOCs, VOC-related and all-cause hospitalizations, before and after voxelotor initiation were compared. Hemoglobin (Hb) responses were evaluated for a subset of patients for whom Hb lab data were available (≥1 Hb value recorded 30 days before initiating voxelotor and ≥1 Hb value recorded after the index date).
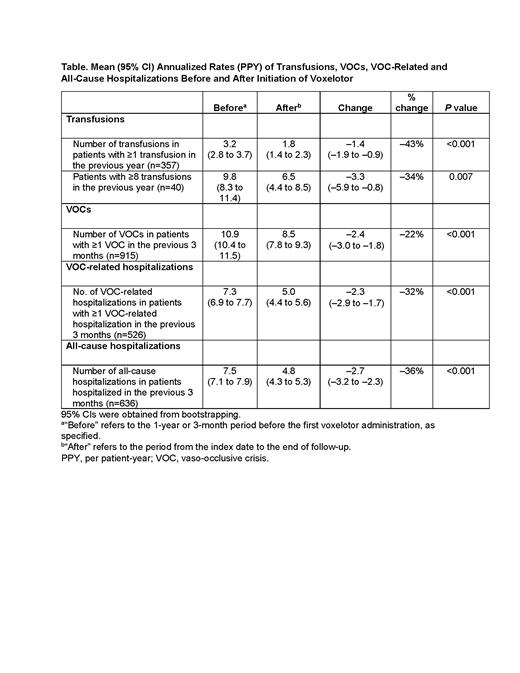
Results: As of March 2021, a total of 2695 eligible patients from the Symphony Health claims database were included in the analysis (mean age: 34.6 years; 60% female); 915 patients (34%) had ≥1 VOC in the 3 months before initiating voxelotor. Among the subset of patients with pre- and post-voxelotor Hb measurements (n=63), mean (95% CI) Hb was 7.9 (7.5-8.2) g/dL at baseline, and final Hb was 8.9 (8.4-9.4) g/dL after voxelotor initiation. Most patients (38/63; 60.3%) achieved a Hb increase of >1 g/dL at any time point during follow-up. In 357 patients who received ≥1 transfusion in the year before voxelotor initiation, the mean (95% CI) transfusion rate decreased by 43%, from 3.2 (2.8-3.7) to 1.8 (1.4-2.3) PPY (P<0.001) (Table). In 40 patients receiving chronic transfusions (≥8), the mean (95% CI) transfusion rate decreased by 34%, from 9.8 (8.3-11.4) to 6.5 (4.4-8.5) PPY (P=0.007). Among patients who had ≥1 VOC in the 3 months before initiating voxelotor, the mean (95% CI) annualized VOC rate decreased by 22%, from 10.9 (10.4-11.5) to 8.5 (7.8-9.3) (P<0.001); and the mean (95% CI) rate of VOC-related hospitalizations decreased by 32%, from 7.3 (6.9-7.7) to 5.0 (4.4-5.6) (P<0.001). In 636 patients who were previously hospitalized in the 3 months before voxelotor initiation, the mean (95% CI) all-cause hospitalization rate reduced by 36%, from 7.5 (7.1-7.9) to 4.8 (4.3-5.3) (P<0.001) after treatment. Limitations of this analysis include the possibility of secular trend biases or general regression to the mean.
Conclusions:In real-world practice, voxelotor increased Hb by ≥1 g/dL, consistent with the HOPE trial. Data suggest statistically significant reductions in transfusions, VOCs, all-cause and VOC-related hospitalizations after voxelotor use. This real-world evidence provides additional support for the use of voxelotor in the treatment of hemolytic anemia and the management of its associated complications.
Shah: Novartis: Research Funding, Speakers Bureau; Emmaus: Consultancy; Alexion: Speakers Bureau; GLG: Consultancy; GBT: Consultancy, Research Funding, Speakers Bureau; Guidepoint Global: Consultancy; Bluebird Bio: Consultancy; CSL Behring: Consultancy. Lipato: Global Blood Therapeutics: Speakers Bureau. Alvarez: Forma Therapeutics: Membership on an entity's Board of Directors or advisory committees; GBT: Membership on an entity's Board of Directors or advisory committees. Delea: Global Blood Therapeutics: Research Funding; Amgen: Research Funding; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding; Takeda: Research Funding; Merck: Research Funding; Policy Analysis Inc.: Current Employment; AbbVie: Research Funding; Novartis: Research Funding; Regeneron: Research Funding; Sanofi: Research Funding. Lonshteyn: Policy Analysis Inc. (PAI): Current Employment; Global Blood Therapeutics: Research Funding; Novartis: Research Funding. Weycker: Policy Analysis Inc. (PAI): Current Employment, Current equity holder in publicly-traded company; Global Blood Therapeutics: Research Funding; Novartis: Research Funding. Nguyen: Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Beaubrun: Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Agodoa: Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal